|
National Transplant Registry
The National Transplant Registry (NTR) collects information about
patients who had undergone organ or tissue transplantation. The
information allows us to estimate the magnitude of transplant activity
in the country. Such information besides being useful to practitioner
of transplantation is also useful in assisting the MOH,
Non-Governmental Organizations, private providers and industry in the
planning and evaluation of transplant services.
The NTR is co-sponsored by the following organizations of the Ministry
of Health Malaysia:
1. National Transplant Coordinating
Committee, MOH
2. Medical Development Division, MOH
3. Malaysian Society of Transplantation
4. Clinical Research Centre(CRC), HKL, MOH
The CRC has established a Transplant Registry Unit to provide the
functional capacity for transplant registration. It maintains the NTR
database.
A Governance Board has been established to oversee the operations of
the NTR. The MOH, Universities, professional bodies, Non-Governmental
Organization and private healthcare providers are represented on this
board. The board's role is to ensure that NTR stay focus on its
objective and be relevant to the needs of its stakeholders.
More about NTR:
 Purpose of the NTR Purpose of the NTR The objectives of Transplant
Registry are:
-
Determine the frequency and distribution of all types of
transplantation activities in Malaysia.
-
Determine the outcomes of transplantation.
-
Determine the factors influencing outcomes of
transplantation.
-
Evaluate transplantation services in the country.
-
Stimulate and facilitate research on transplantation and its
management.
Back to top
 Organization Chart of
National Transplant Registry Organization Chart of
National Transplant Registry
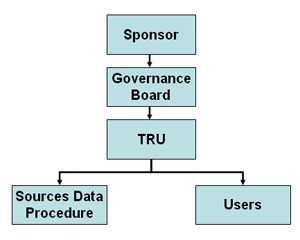
Sponsor
The current sponsors of the NTR are:
• National Transplant Coordinating Committee
• Medical Development Division, MOH
• Malaysian Society Of Transplantation
• The Clinical Research Centre, HKL
Sponsors shall:
• Provide leadership and direction for the NTR
• Establish a Governance Board (see below) to govern the NTR.
All members of the Governance Board are appointed by sponsors.
Membership however should represent all interested parties,
which include source data producers, users and representative
from the designated collaborating unit.
• The designated collaborating unit shall provide all the
financial, human and technical resources required, if necessary
with financial contribution from industry or donor agencies.
Governance Board
The Governance Board shall be established by sponsors to
oversee the operations of registry. Interested parties including
source data producers, TRU and target groups or users are
represented on this Board.
Transplant Registry Unit (TRU)
The collection, and analysis of data, and feedback of
information are performed in a single centre referred to as the
TRU. This unit is staffed by CRM, CRA, epidemiologist,
statistician, information technology personnel and other
supporting staff. The Clinical Research Centre (CRC) is
currently the designated TRU.
Source Data Producers
The NTR receives data on organ/ tissue transplantation
from 3 main sources:
1. The individual doctors who provide transplantation services,
who voluntarily report data to the NTR. Data collection will be
from six main types of transplantation services:
• Blood and marrow Transplant
• Heart and Lung Transplant
• Liver Transplant
• Renal Transplant
• Cornea Transplant
• Bone / Tissue Transplant
2. The National Vital Registration system (Jabatan Pendaftaran
Negara). These data are useful for determining or verifying
mortality outcomes of transplant patients.
3. Information Documentation Unit of the MOH, which operates the
Health Management Information system (HMIS).
Users
These are the individuals or institutions to which the
regular registry reports are addressed.
They include
• Transplant professional
• Health care provider
• Public health practitioner
• Industry
• Decision maker
• Researcher
• Press and public
It is their needs for information to assist in planning and
managing transplant activities that justify the investment in
this registry.
Back to top
 Sources of Transplant Data in Malaysia Sources of Transplant Data in Malaysia
The NTR receives data on transplantation from three main sources:
1. The individual doctors who provide transplantation services, who
voluntarily report data to the NTR. The data collected will be
systematic and standardized. Data collection will be from six main
types of transplantation services:
2. The National Vital Registration system (Jabatan Pendaftaran
Negara). These data are useful for determining or verifying
mortality outcomes of transplant patients.
3. Information Documentation Unit of the MOH, which operates the
Health Management Information system (HMIS).
Back to top
 Wouldn’t you want to report to the NTR? Wouldn’t you want to report to the NTR?
Click here for listing of NTR participants
Click here to register online For the NTR to succeed, ideally all health professionals who have
anything to do with transplantation ought to report to the NTR. Unlike
communicable diseases, transplantation however is not a reportable
condition. We urge you to do your part for our community, and help NTR
obtain crucial information for healthcare development in this
country especially in the field of organ and tissue transplantation.
 What are the benefits of participating in NTR? What are the benefits of participating in NTR?
Apart from doing your bit for our community, here are some other
benefits of participating in NTR:
-
Invitation to all functions organized by the NTR.
-
Acknowledgement in all publications of the NTR.
-
Personal copy of all NTR publications free of charge.
-
Free listing in the “Directory of Transplant Services in Malaysia”, an
annual publication by NTR.
-
Free listing in the NTR’s web site.
-
Tap into a network of like-minded people from diverse professional
disciplines and backgrounds.
Back to top
 What about confidentiality? What about confidentiality? Current legislation allows doctors to release their patients’ data to
persons demonstrating a need, which is essential to public health and
safety. The NTR meets this requirement.
The NTR have also developed strict information security policies and
procedures to protect data confidentiality in accordance with standard
disease registration practice and in compliance with professional
standard and applicable regulatory requirements.
Back to top
|
